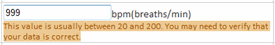|
BENEFITS OF ePASS
To 100% customer satisfaction
Speedy EDC system startup
The ePASS provides coding modules. There is no need to wait for a month to create validation edits. Library of eCRFs are saved and re-used it by administrator, which is affording faster trial set-up for future studies.
User-Friendly Interface Design
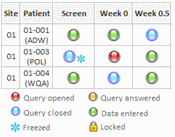
The ePASS’s user interface is very user-friendly. Data can be entered in a few clicks. All necessary information or action is indicated by symbols, providing a look and feel that is very familiar to site users.
Cost competition
The ePASS offers to clients reasonable, cost effective pricing.
ePASS REGULATORY AND SECURITY SUMMARY
Regulatory Summary
- Electronic audit trails of all changes and activity to clinical study data
- 128-bit Data Encryption
- Access restrictions to clinical study data
Security Summary
- Encryption: All clinical study data into ePASS’s servers are encrypted. Secure Sockets Layer (SSL) certificate serves that allows data to be transmitted over secure networks.
- Ownership: ePASS does not own any data entered by its end user.
- Access: Only user with usernames and passwords assigned by the administrator has access to the data.
Monitoring and Optimization
The system (any HTTP& HTTPs including IP address) is continuously tested optimal performance, security and reliability at regular intervals.
Redundancy and Disaster Recovery
Daily backups of ePASS server are made as a part of regular system maintenance and are kept for a year. The system backups and restore is quick and own local backup or restore from system generated backups within a few minutes.
|